By Myron Levin and Stuart Silverstein
FairWarning
For several years, doctors and medical spas around the country have touted a fat-melting device called the LipoTron 3000, or Lipo-Ex, as a revolutionary way for people to slim down.
Signature Medical Spa in Tampa, Fla., in an online pitch for its “Lipo-Ex Spring Fling Fat-Off!,” described the technology as “truly the only non-invasive way to reduce fat.”
Praise also came from Sculpt Medical Spa in Chicago, which called the procedure “the most innovative, effective, and technologically advanced” non-surgical method of removing fat.
Doctors have appeared on TV news shows in Houston, Phoenix and Miami to promote LipoTron treatments.
These testimonials have translated into millions of dollars in sales for physicians, med spas, and the device’s manufacturer, RevecoMED International of Fullerton, Calif.
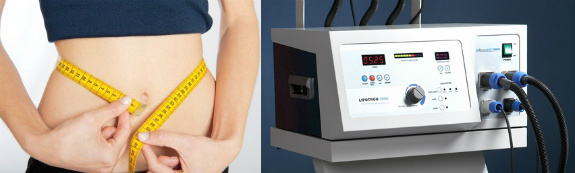
But there’s a problem: The LipoTron, which targets fat with radiofrequency waves, has never been cleared or approved by the U.S. Food and Drug Administration, which would make it illegal under federal law to sell or promote it for weight loss.

Paige Peterson told FairWarning she had made 39 LipoTron sales, even though she was aware the device had not been cleared by the FDA.
The FDA is aware of the activity. But an investigation by FairWarning found that the agency has not taken enforcement action—even though it has known about the situation at least since January, 2010. At that time, two whistleblowers, one a former LipoTron distributor, provided sales records and a trove of other documents to an FDA criminal investigator.
The case spotlights the booming, multi-billion-dollar business of aesthetic medicine—and the willingness of some doctors and med spas to use unapproved devices as they vie to be first with the latest technologies to smooth wrinkles, tighten skin and sculpt the body.
The FDA won’t say if it is investigating Reveco, citing a policy not to discuss investigations or acknowledge if there is one.
For his part, RevecoMED President James S. Rosen said the agency hasn’t contacted the company. He asserted that, “As of today, we are compliant with the FDA.”
Still, for observers such as Dr. Patricia K. Farris, a clinical associate professor of medicine at Tulane University and a spokesperson for the American Academy of Dermatology, the situation is baffling.
Told of the unauthorized sales, Farris responded: “It shocks me the FDA would not have cracked down on them.”
“I mean, radiofrequency is an electrical device, and you can’t just be throwing these things in the marketplace without doing the right studies to make sure that, A, the device is safe and, B, that the thing does something and has some benefit.”
Dr. Suzanne Yee, a Little Rock, Ark., plastic surgeon whom Reveco asked several years ago to take part in a LipoTron study, said she was surprised to learn that the company already was selling the device.
She noted that some medical spas have falsely stated on their websites that the LipoTron is FDA-approved. “It’s not FDA-approved,” Yee said. “I think that’s dishonest.”
There have been scattered incidents of patients receiving minor shocks and burns from LipoTron treatments, but no known reports of serious injury.
While the FDA has failed to act, the Texas Department of State Health Services issued a warning letter last September to a Fort Worth distributor for marketing the LipoTron without FDA clearance. According to an agency report, Mark Durante, managing partner of Advanced Aesthetic Concepts, told state investigators that the LipoTron had been cleared by the FDA, but later corrected himself to say paperwork had been filed but no clearance yet given.
Durante told FairWarning that, in response to the warning, his company changed some language on its website. However, a spokeswoman for the Texas agency said it recently opened a second complaint investigation of Advanced Aesthetic Concepts.
Selling for as much as $85,000, the LipoTron passes radiofrequency waves through the body to heat, and destroy, fat cells. According to Reveco, the procedure targets subcutaneous fat, which is just below the skin, as well as visceral fat surrounding the vital organs, but without harming adjacent tissues. Spas typically recommend six to eight treatments for about $400 each.
According to interviews and records, Reveco first sought a green light from the FDA in 2007. It chose the FDA’s market clearance procedure, which is less demanding than the formal approval process.
To get a new device cleared this way, the manufacturer must show it is similar in safety and effectiveness to products that are already on the market.
However, Reveco’s bid failed. The company’s initial application “wasn’t in-depth enough,” Rosen said, and the FDA repeatedly sought additional data. Finally, according to Rosen, “We said, ‘You know what, it’s not worth it.”
According to interviews and a document reviewed by FairWarning, the FDA then told Reveco that the device could not be marketed.
LipoTron sales continued, however. Rosen wouldn’t disclose how many of the devices have been sold, but the number is believed to be in the low hundreds.
In 2011, Reveco took another tack with the FDA. It classified the LipoTron as a massager used for relief of minor pain. That would make it, in FDA parlance, a Class 1 device — a category that includes such simple, low-risk items as elastic bandages and examination gloves.
The advantage for Reveco is that massagers can be sold without a green light from the FDA. They automatically are exempt from FDA review and can be put on the market once a notice is filed.
Yet doctors and med spas have been promoting the device on the Internet not for massages but for removing fat.
Rosen said that was not Reveco’s responsibility, stating that the company can’t dictate what doctors do or “police everything out on the Internet.”
Asked who would pay $85,000 for a massager, Rosen replied: “Anybody that wants to buy it.”
Physicians are free under federal law to prescribe unapproved, or “off-label,” uses of drugs or medical devices—but only if the products have been cleared or approved for another purpose, according to the FDA.
FDA spokeswoman Sarah Clark-Lynn said in an email that if a device is not legally on the market, “a physician should not have been able to obtain it, much less use it on a patient.”
Dr. Sherwood Baxt, a New Jersey plastic surgeon who advertised the procedure in a promotional video, said that when he bought the LipoTron he wasn’t troubled by its lack of FDA clearance. He explained that he had used unapproved devices before and, while he considered the agency’s green light a marketing advantage, he didn’t consider it necessary.
Besides, Baxt said, “We were told FDA approval was imminent.” It didn’t work out that way, however, and, he said, “After two years, I just stopped asking.”
He continues to use the device for skin tightening on certain patients but quit using it for fat reduction. For fat reduction, Baxt said, “it wasn’t as effective as I thought it was going to be.”
The FDA was informed of the unauthorized sales through an anonymous call. Paige Peterson, a former LipoTron distributor, and Belinda W. Worley, a marketing consultant who worked with her, told FairWarning they dialed in from a hospital phone in hopes the call could not be traced.
But they agreed to meet with criminal investigator Evan Rae a few days later at a Hilton inn in Waco, mid-way between Rae’s office in Austin and Dallas, where Peterson and Worley lived.
They found a quiet spot in the lobby bar, which was closed in the morning, and talked for a couple of hours. Peterson said she gave Rae a detailed statement, a computer flash drive and copies of records, including emails, memos and invoices. Rae taped the conversation and snapped photos of the LipoTron 3000 the women had brought along. Rae declined to be interviewed.
Peterson told FairWarning she had made 39 LipoTron sales, even though she was aware the device had not been cleared by the FDA. The evidence she gave Rae “was just as damning of me as everybody else. I have zero assurances that the FDA is not going to arrest me.”
Peterson admitted there was no love lost between her and Reveco. She said she had paid out-of-pocket for some research costs aimed at getting FDA approval, but had not been reimbursed. And she said the company dumped her as a distributor in favor of another sales group.
But Peterson also said Reveco had misled her with repeated assurances it was taking all proper steps and FDA approval was imminent—and spread this misinformation to some anxious customers.
“I had run out of acceptable answers to give the doctors that had purchased the LipoTron,” she said. “I needed to fall on my sword and tell the truth.” Better to come clean, Peterson decided, than to wait for the FDA “to come knocking on my door.”
While declining to comment on Peterson’s statements, Rosen said she had gone over to “the dark side.”
“She’s a person that’s vindictive,” he said. “She’s doing it out of spite.”
For her part, Peterson says that after 2½ years she is surprised and frustrated by the apparent lack of action.
“Why do we have an FDA?” she asked.
“I tried to do what I thought was right, and nobody’s doing anything about it. Everybody gets to thumb their nose at the law.”
FairWarning is a nonprofit, online investigative news organization focused on safety and health issues.